Introduction
The public health response to human immunodeficiency virus (HIV) has drastically evolved over the past two decades. Twenty years ago, annual HIV infections were over 2.5 million, and acquired immunodeficiency syndrome (AIDS) related deaths peaked at 2.1 million globally. Access to costly antiretrovirals (ARVs) was limited, particularly in resource-constrained regions. In sub-Saharan Africa, the epidemic’s impact was dire, with child mortality tripling and life expectancy plummeting by 20 years. [1][2] Since then, collaborative efforts between governments, the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), the Global Fund, and other partners have completely transformed HIV treatment accessibility worldwide. [1] In 2023, according to The Joint United Nations Programme on HIV/AIDS (UNAIDS) Global HIV Statistics, 39.9 million people were living with HIV, 1.3 million newly acquired HIV, and 630 000 people died from AIDS-related illnesses (Table 1). [2] While great progress has been made towards fighting HIV/AIDS, more work is needed. In this Insight Article, we explored the current clinical and economic landscape focused on Lenacapavir in HIV prevention and treatment.
Table 1. HIV/AIDS Statistics 2000 – 2023
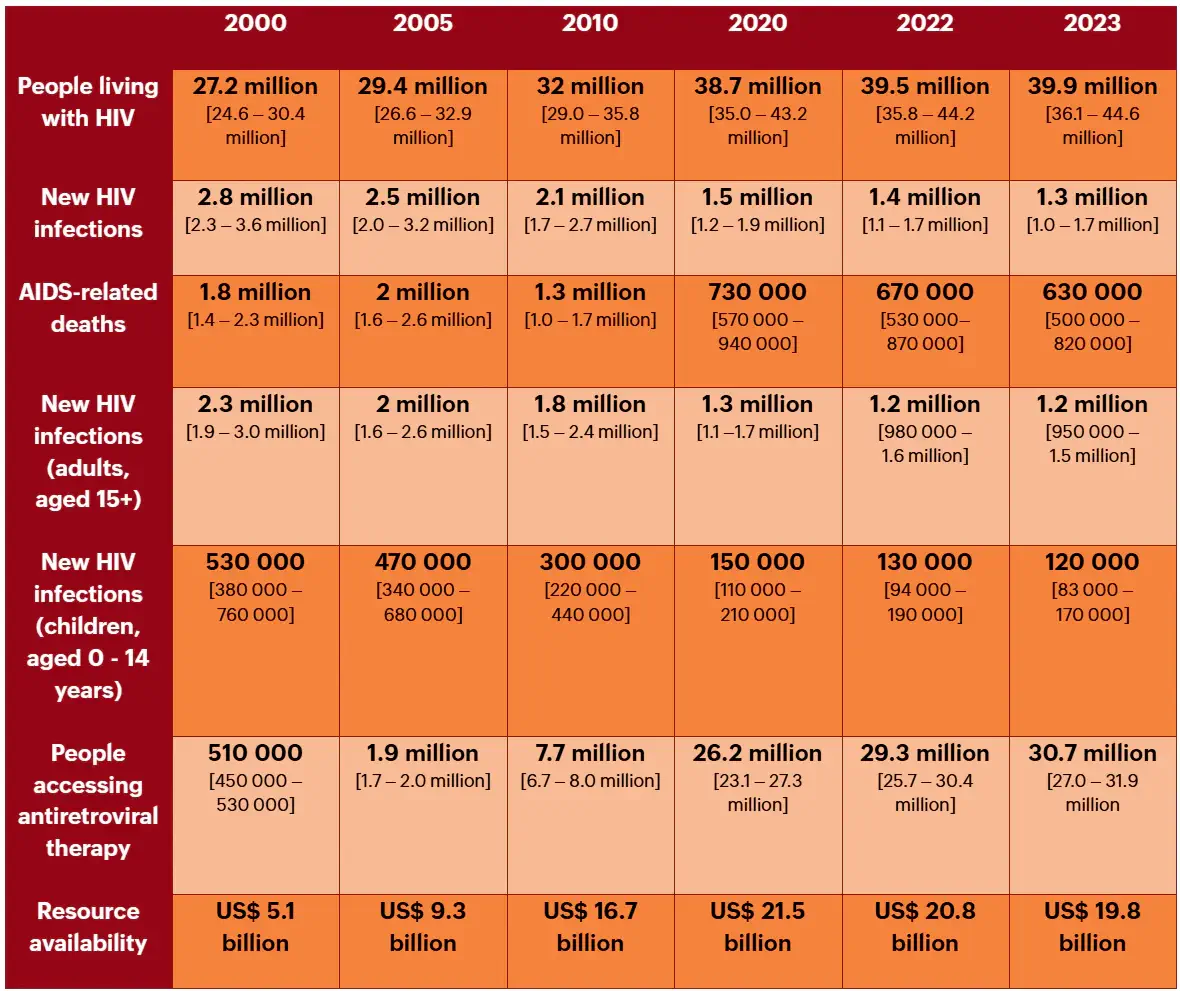
AIDS = acquired immunodeficiency syndrome; HIV= human immunodeficiency virus.
The Clinical Landscape of HIV Preventative Treatment
Studies have shown the exceptional effectiveness of daily oral ARVs for HIV prevention among diverse populations. Currently, a fixed-dose combination of emtricitabine-tenofovir disoproxil fumarate (F/TDF) and emtricitabine-tenofovir alafenamide fumarate (F/TAF) are the dominant oral pre-exposure prophylaxis (PrEP) options on the market. [1][4]
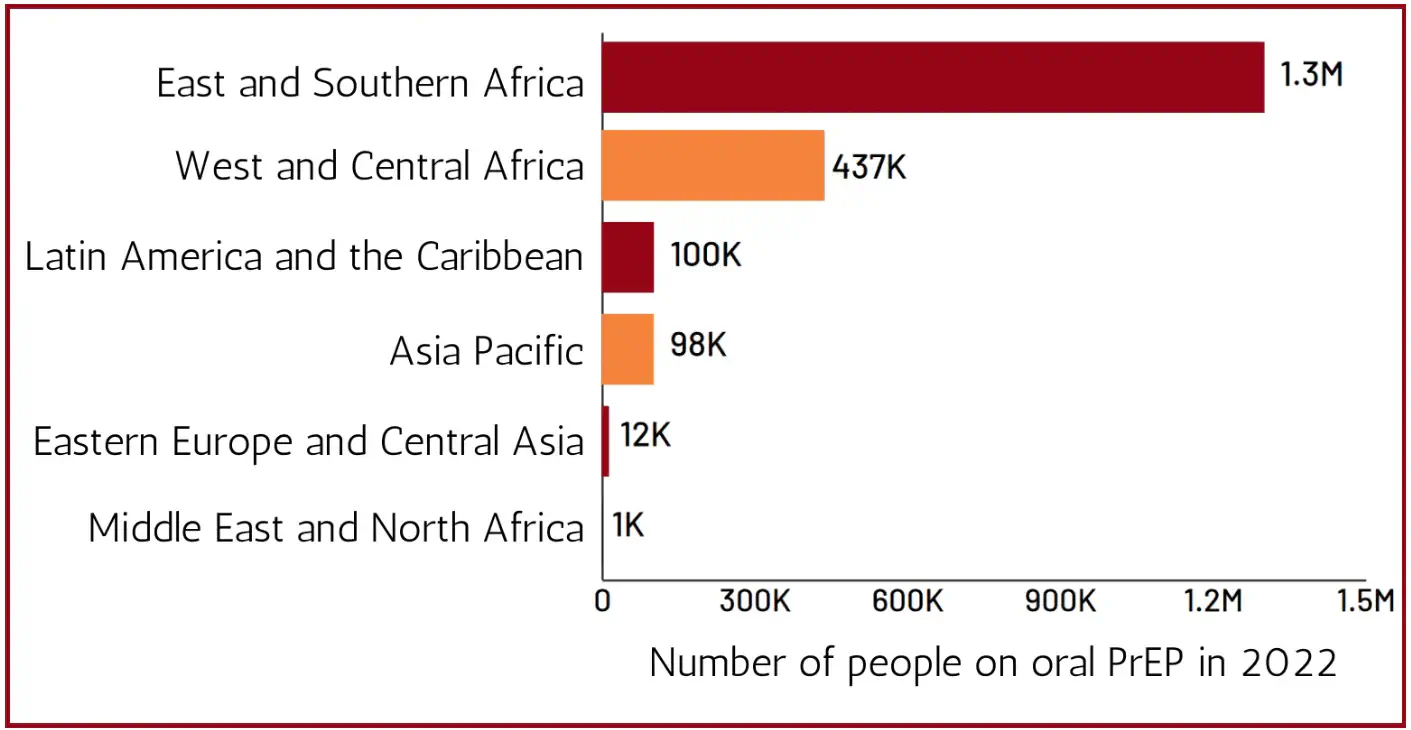
While oral PrEP initiations are on the rise, its availability in low- and middle-income countries (LMICs) remains limited to a few nations, predominantly in sub-Saharan Africa. Despite most new HIV infections occurring in sub-Saharan Africa, a significant portion arises from Asia and the Pacific, where PrEP coverage is notably lower (Figure 1). National programmes in these regions can improve this by expanding oral PrEP usage among vulnerable groups. [1] However, improving access to PrEP is not an all-encompassing solution.
Challenges in Adherence to Oral PrEP
A recent global systematic review and meta-analysis revealed that 41% of individuals using oral PrEP discontinued treatment within 6 months. Individuals under 30 display lower adherence rates due to clinical issues like side effects, logistical hurdles such as storage and stock shortages, and social obstacles like stigma and violence. [3] With over 2.5 million individuals receiving oral PrEP in 2022, it is important to consider a person-centred approach that integrates the individual risk profiles of PrEP users. Combination prevention approaches need to be holistic, integrating socio-behavioural and biomedical interventions. [1]
A study in Kenya revealed that most PrEP discontinuations were due to changing risk profiles, such as new partner situations or alternative prevention methods. Achieving real-world success may require engaging individuals during high-risk periods, allowing them to use PrEP intermittently as necessary. [3] Expanding PrEP choice should encompass various HIV prevention tools, such as condoms, male medical circumcision, and education on treatment as prevention. Flexibility between methods and where and how PrEP is accessed, whether through traditional health facilities or community-based services, is key. Integrating PrEP choice with other sexual health services like contraception and sexually transmitted infections (STI) testing would enhance overall care. The goal is to empower individuals to customise their prevention strategies that align with their preferences. [3]
While prevention and treatment expansion has curbed HIV transmission rates, new infections persist, indicating that current strategies alone are insufficient to reach epidemic control. To address these challenges and enhance HIV prevention in adolescents and young adults, new longer-acting PrEP options such as implants and injectables are promising. [3]
Lenacapavir in HIV prevention and treatment
Gilead Sciences recently unveiled unprecedented results from the PURPOSE 1 study, showcasing 100% efficacy in preventing HIV infections among 2134 young women and adolescent girls in South Africa and Uganda who received twice-yearly injections of long-acting lenacapavir as PrEP. [4] These results prompted the premature end of the randomised phase of the trial, as lenacapavir significantly outperformed daily oral PrEP medications in the study (Figure 2 – Lenacapavir in HIV prevention and treatment).
The study’s design involved comparing infection rates in the lenacapavir arm with the background HIV incidence in the community, demonstrating zero infections with lenacapavir compared to standard of care oral PrEP. Despite this remarkable achievement, the primary challenge lies in ensuring affordable access to lenacapavir. The true potential of long-acting injectables is hindered by low production capacity, patent issues, and a lack of strategic pricing strategies that address the high costs of these medicines. [1][4][5]
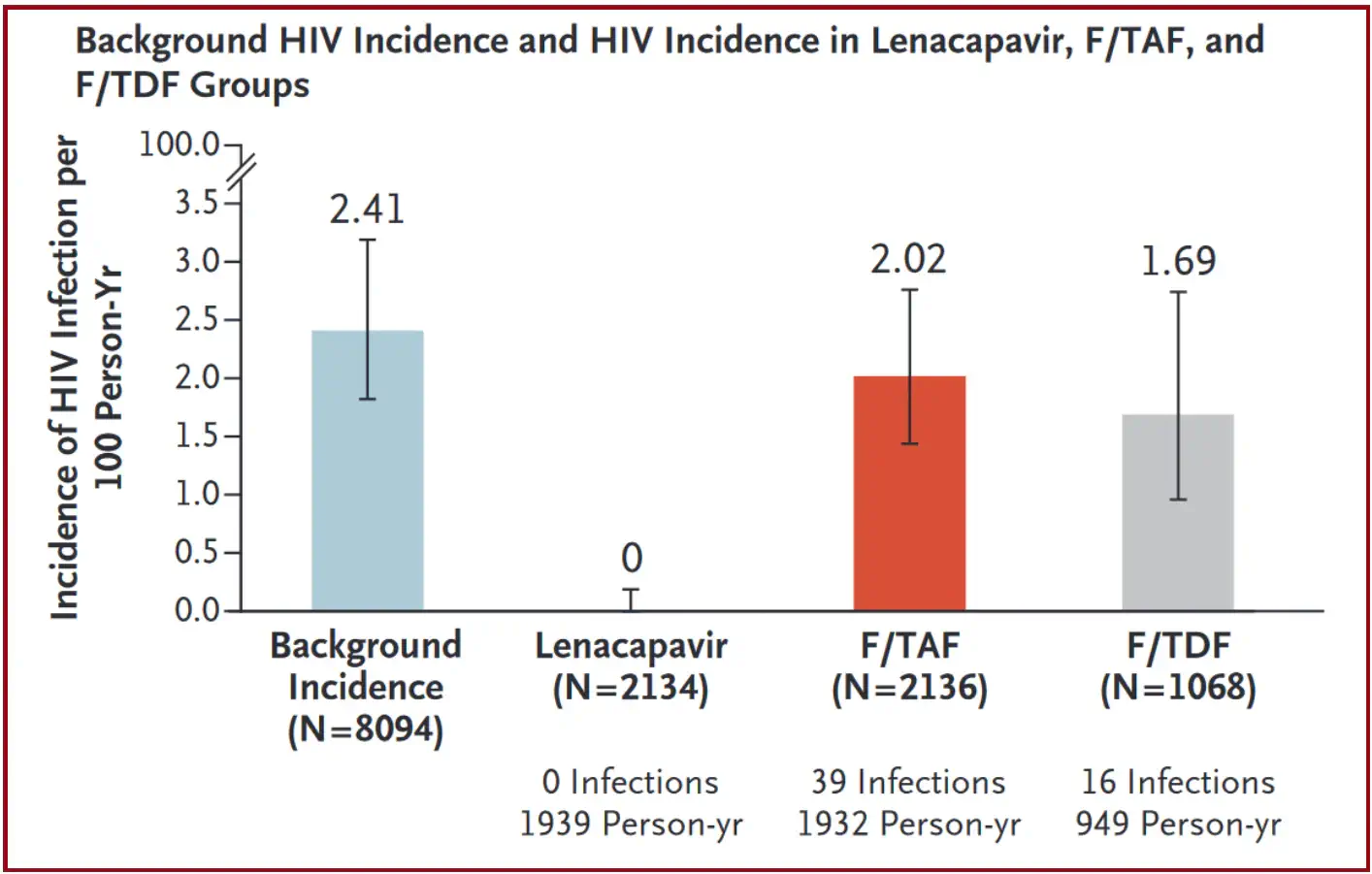
Lenacapavir reduced HIV incidence by 100% as compared with background HIV incidence and by 100% as compared with F/TDF. HIV incidence with F/TAF did not differ significantly from background HIV incidence, and there was no significant difference in HIV incidence between F/TAF and F/TDF.
F/TAF = emtricitabine–tenofovir alafenamide; F/TDF = emtricitabine–tenofovir disoproxil fumarate; HIV = human immunodeficiency virus.
An Age-old Challenge: Affordable Access
Significant progress in HIV treatment accessibility was facilitated by the emergence of affordable generic ARVs. Presently, generic dolutegravir (DTG) has widespread use in LMICs, costing less than US$45 per person annually. Notably, paediatric DTG is now accessible to 160,000 children yearly, thanks to strategic pricing agreements. [1]
The ARV market in LMICs remains robust, estimated at US$1.9 billion in 2022. Concurrently, testing advancements have decentralised HIV services, with affordable point-of-care options like HIV self-tests emerging, albeit with limited uptake. [1] Unfortunately, gaps still persist in treatment, such as patient retention challenges and slower adoption of second-line darunavir/ritonavir (DRV/r) due to high costs, limited availability, and logistical barriers. The true potential of long-acting injectables is similarly hindered by low production capacity, patent issues, and a lack of strategic pricing strategies that address the high costs of these medicines. [1][ 5]
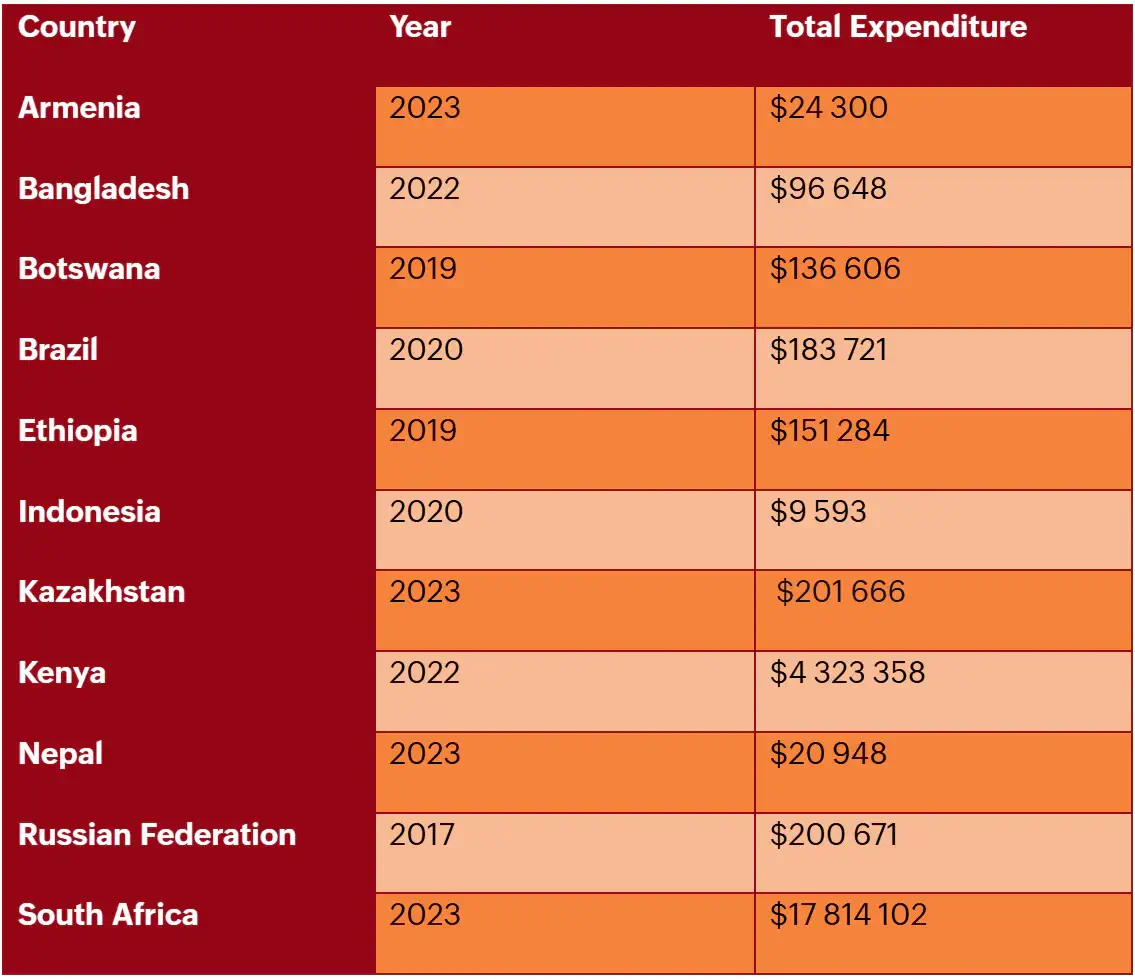
Lenacapavir is currently licensed in the US as treatment for drug-resistant HIV, under the brand name Sunleca with a list price of $40 000 a year. Generic oral PrEP can be obtained for less than 1% of that price, [5] making lenacapavir unaffordable for both upper- and lower-income countries. In many countries, the cost of lenacapavir is more than their entire PrEP budget (Table 2).
Table 2. Country Reported Expenditures on PrEP
Economic Barriers to Lenacapavir Accessibility
Research by Meyer-Rath et al. focused on the affordability and accessibility of another injectable ARV, long-acting cabotegravir (CAB-LA). Based on this research, lowering the price through increased global rollout can enhance affordability and widen access to lenacapavir. This would mirror previously successful strategies to reduce costs and expand availability of essential medications. [7]
Generic manufacturing companies can produce lenacapavir for approximately $40 per year, similar to oral PrEP costs. [8] HIV community organisations in Argentina, India, Thailand, and Vietnam, have already filed oppositions against Gilead’s patent for lenacapavir. Gilead plans to produce the drug at scale for affordable prices in HIV-affected, resource-limited nations. This move aims to secure access until generic versions are available, which is crucial for HIV prevention efforts in high-burden regions. [8]
Historical Examples
Historical examples demonstrate the effectiveness of enabling broader access to drive down costs and facilitate widespread distribution of life-saving drugs. This approach, coupled with support from entities like PEPFAR, the Global Fund, and Unitaid, played a key role in making HIV treatment globally accessible through fostering generic competition and shaping the market. Leveraging similar mechanisms may be essential in ensuring that lenacapavir reaches those most in need, highlighting the importance of collaborative efforts to address pricing challenges and promote equitable access to innovative treatments. [7]

Once long-acting injectables are affordable, there are still anticipated challenges for administering these treatments outside the clinical setting. Several ongoing implementation studies are exploring the practical delivery and demand generation strategies for various PrEP products such as oral PrEP, CAB-LA, and lenacapavir. [3] Figure 4 below illustrates the ease of integrating a specific intervention into the existing healthcare framework, with respect to various criteria.
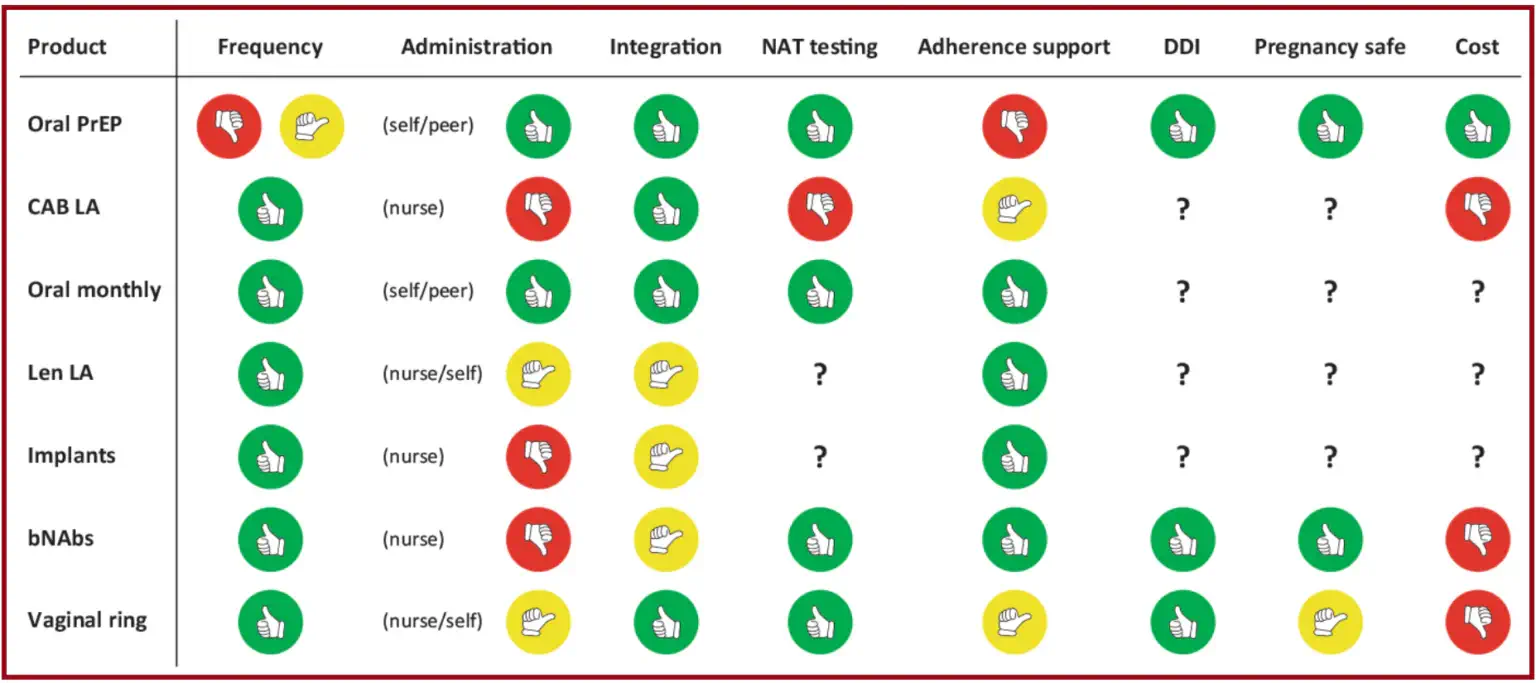
Green indicates that the intervention is optimal/well-supported for the specific criteria. Yellow indicates that the intervention is not optimal/requires further improvement for the specific criteria. Red indicates that the intervention is not suitable or requires significant improvement for the specific criteria.
PrEP = pre-exposure prophylaxis; CAB-LA = long-acting cabotegravir; Len LA = long-acting lenacapavir; bNAbs = broadly neutralising antibodies; NAT = nucleic acid test; DDI = drug-drug interaction.
Quality of Life: The Hidden Costs of Daily Dosing
People living with HIV (PLWH) face a range of emotional and psychosocial challenges, including coping with diagnosis, treatment decisions, social support, and workplace issues. Stigma significantly impacts the well-being and willingness to access healthcare in PLWH. Moreover, PLWH have unique treatment needs, with antiretroviral therapy influencing their quality of life in complex ways – the emotional toll of managing HIV treatment, including pill fatigue and lifestyle adjustments, can significantly disrupt the lives of PLWH, reshaping their daily routines, relationships, and outlook on life. [9] Similar challenges may be experienced by those on oral PrEP.
According to the 2019 Positive Perspectives survey of PLWH across 25 different countries, 33% of participants felt stressed when having to take daily pills, and 38% were concerned that daily pills would increase the likelihood of people around them finding out their HIV status. Some individuals felt empowered in taking their medication and were more likely to adhere to treatment, while others were less likely to adhere if they felt daily dosing hindered their day-to-day life. [9] Long-acting injectables such as lenacapavir could significantly reduce the stress associated with daily dosing and improve quality of life in these individuals.
Key Takeaways: Lenacapavir in HIV prevention and treatment
Progress has been made in reducing HIV transmission and improving treatment access, yet challenges remain in achieving epidemic control. Breakthrough drugs like lenacapavir offer an opportunity to upgrade HIV prevention efforts, but ensuring affordable access, particularly in high-burden regions, remains a significant hurdle to overcome.
Addressing the affordability of drugs like lenacapavir requires a multi-faceted approach, including leveraging generic competition, advocating for strategic pricing strategies, and fostering global partnerships to facilitate widespread distribution.
Considering the hidden costs of daily dosing on the quality of life of individuals living with HIV is also key. Long-acting injectables can alleviate the burden of daily medication regimens and improve overall well-being.
By addressing not just the medical aspects but also the psychosocial and emotional challenges faced by PLWH, we can ensure a more holistic approach to HIV care that prioritises the individual needs and experiences of those affected by the condition.
Ongoing efforts are vital to sustain progress, reduce mortality rates, and enhance access to comprehensive care globally. In the case of HIV, with the tools we have available today, prevention is truly better than cure.
References
[1] CHAI HIV Market Report, Issue 14, October 2023 [Internet]. [cited 2024 July 21]. Available from: https://www.clintonhealthaccess.org/report/2023-hiv-market-report-the-state-of-hiv-market-in-low-and-middle-income-countries/
[2] UNAIDS Fact Sheet 2024, Global HIV Statistics [Internet]. [cited 2024 July 21]. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
[3] Pike C, Rousseau E, Bekker LG. Promises and potential pitfalls of long-acting injectable pre-exposure prophylaxis. South Afr J HIV Med. 2023 Jul 27;24(1):1497. doi: 10.4102/sajhivmed.v24i1.1497. PMID: 38089889; PMCID: PMC10713495
[4] Bekker LG, Das M, Abdool Karim Q, Ahmed K, Batting J, Brumskine W, et al; PURPOSE 1 Study Team. Twice-Yearly Lenacapavir or Daily F/TAF for HIV Prevention in Cisgender Women. N Engl J Med. 2024 Jul 24. doi: 10.1056/NEJMoa2407001. Epub ahead of print. PMID: 39046157.
[5] No HIV infections seen in groundbreaking trial of twice-yearly injectable PrEP [Internet]. [cited 2024 July 21]. Available from: https://www.aidsmap.com/news/jun-2024/no-hiv-infections-seen-groundbreaking-trial-twice-yearly-injectable-prep
[6] UNAIDS HIV Financial Dashboard [Internet]. [cited 2024 July 24]. Available from: https://hivfinancial.unaids.org/hivfinancialdashboards.html#
[7] Meyer-Rath G, Jamieson L, Pillay Y. What will it take for an injectable ARV to change the face of the HIV epidemic in high-prevalence countries? Considerations regarding drug costs and operations. J Int AIDS Soc. 2023 Jul;26 Suppl 2(Suppl 2):e26106. doi: 10.1002/jia2.26106. PMID: 37439062; PMCID: PMC10338998.
[8] AIDS Activists Delight in HIV Prevention Trial Results – And Press Gilead to Lower Price of ‘Miracle Drug’ [Internet]. [cited 2024 July 29]. Available from: https://healthpolicy-watch.news/aids-conference-delights-in-hiv-prevention-trial-results/
[9] de Los Rios P, Okoli C, Castellanos E, Allan B, Young B, Brough G, et al. Physical, Emotional, and Psychosocial Challenges Associated with Daily Dosing of HIV Medications and Their Impact on Indicators of Quality of Life: Findings from the Positive Perspectives Study. AIDS Behav. 2021 Mar;25(3):961-972. doi: 10.1007/s10461-020-03055-1. Epub 2020 Oct 7. Erratum in: AIDS Behav. 2021 Sep;25(9):3045. doi: 10.1007/s10461-021-03206-y. PMID: 33026574; PMCID: PMC7936969.
Table of Contents