Introduction
Haemophilia A, is a recessive bleeding disorder which causes lifelong bleeding due to reduced or nonexistent circulating factor VIII (FVIII) activity. [1] Haemophilia is classified into three types: Haemophilia A and Haemophilia B, which are inherited through the X-linked route, and Haemophilia C, which is inherited through the autosomal recessive mode. [2] According to the annual survey conducted by the World Federation of Haemophilia (WFH), it is estimated that there are 429,232 individuals worldwide who suffer from haemophilia. [3cce] Nearly 85% of all haemophilia cases are associated with this haemophilia A. Based on FVIII levels, haemophilia is categorised as mild (6-40%), moderate (1-5%), and severe (<1%). [1]
The burden of this disease has a significant impact on the healthcare system, particularly in low- and middle-income countries (LMICs) due to the recurrent and costly treatment as well as the interventions needed due to the damage and effects caused by this disease. In this Insights Article we’ll explore the economic and clinical landscape of haemophilia A and provide recently published results on the cost-effectiveness of treatment options.
Clinical Landscape of Haemophilia A
Haemophilia A is a genetic disorder that affects males and females differently and is seen in approximately 1 in 5,000 male births. While men tend to be symptomatic and have spontaneous bleeding episodes, females may often be the carriers but be asymptomatic. [1] Individuals with severe haemophilia A have a greater probability to experience significant and potentially life-threatening bleeding as a result of trauma and surgical procedures. Joint thrombosis, as well as other forms of spontaneous bleeding in muscles, soft tissues, and joints, can cause long-term impairment or disability and diminished quality of life. [1][4]
The diagnosis of haemophilia includes the suspicion due to familial history, clinical manifestations as well as laboratory testing. [5] Genetic testing is rapidly becoming more important in the diagnosing phase as it allows for more tailored clinical management. Inhibitors against FVIII or FIX cause difficulties in haemophilia care. Genetic risk factors for inhibitor development are largely due to the gene variation and there is a well-established link between genetic variation and inhibitor development. [6]
Table 1: Summary of bypassing and non-factor replacement therapy for Haemophilia A [4]
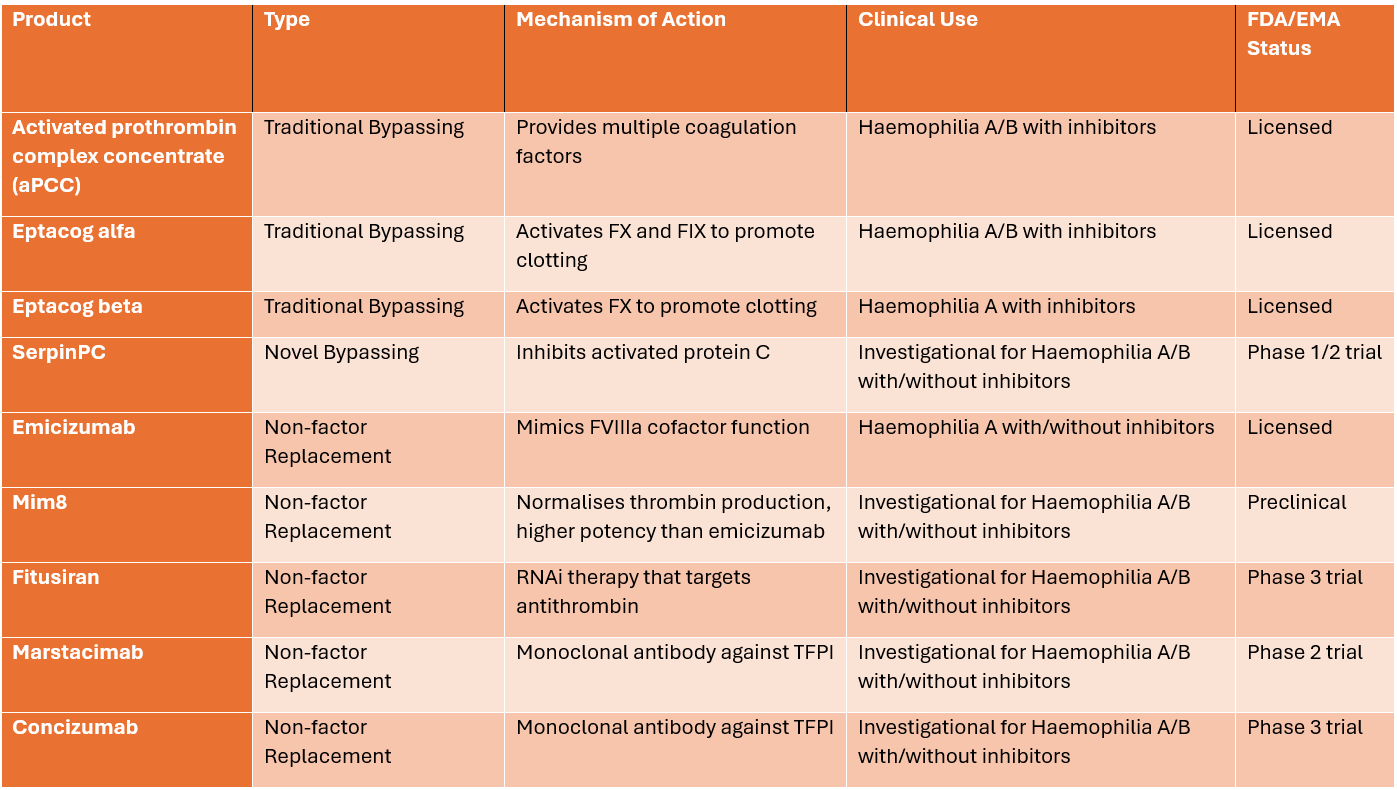
Please note that the status column refers to the regulatory status in the United States or the European Union, except for Concizumab, which has been approved in Canada for haemophilia B as of March 2023. The clinical use and approval status are subject to change as new data emerge and regulatory decisions are made.
Current Treatment Landscape for Haemophilia A
The standard of care for patients with severe haemophilia A is the administration of intravenous factor-VIII concentrates, which can be derived from plasma or produced through recombinant technology. It is given in response to bleeding (referred to as “on-demand therapy”) or to avoid bleeding (referred to as “prophylactic therapy”). Nevertheless, approximately 20-30% of individuals with severe haemophilia A experience the development of inhibitors against factor-VIII. [1] Novel pharmacological therapy for haemophilia have been developed, using various ways to produce hemostasis with or without inhibitors. These include extending the half-life of recombinant clotting factors, mimicking factor VIII cofactor activity, rebalancing coagulation by blocking natural anticoagulants, and promoting endogenous clotting factor production through gene therapy. [7][4]
Emicizumab is one such novel treatment and was designed to avoid repeated intravenous administration of FVIII and also to avoid breakthrough bleeding. This treatment option is associated with a high cost and mostly only practiced in developed countries. [8] Worldwide availability to emicizumab is substantially correlated with per capita income: 42.1% in high-income nations, 24.3% in upper middle-income, 25.2% in lower middle-income, and 7.5 percent in low-income. [9] Humanitarian aid for the use of emicizumab is seen in developing countries around Africa, Latin America, Asia and Eastern Europe. [8][9]
Economic Landscape of Haemophilia on Healthcare Systems
Treatment for haemophilia does come at a high cost as the direct cost of bypassing agents alone account for 98% of all the healthcare costs in severe haemophilia A. Adherence and treatment effectiveness are affected by the high costs of bypassing agents. [4] Other direct costs include hospitalisation, resource utilisation, and diagnostic expenses. Indirect costs are caregiver expenses, absenteeism from school or work and productivity loss. [3] Emicizumab has become particularly appealing for access to haemophilia treatment in LMICs due to the lack of specialised treatment centres, the ease of once per month subcutaneous dosing and the possibility of home dosing and storage. Many papers have examined LMIC nations’ efforts to minimise conventional and approved emicizumab dosage regimens in order to lower overall treatment cost and enhanced access to care. The first report from Malaysia was presented in 2021. [9]
Cost-Effectiveness of Haemophilia Treatments in LMICs
Recognising the clinical efficacy of emicizumab, India a LMIC, approved its use in April 2019. Despite the clear therapeutic benefits, the economic implications of adopting emicizumab in a resource-constrained healthcare environment like India’s have not been thoroughly evaluated. Given the substantial burden of haemophilia on patients and healthcare systems in LMICs, it is imperative to assess the cost-effectiveness of emicizumab. [1][3]
In India, the cost of prophylactic emicizumab treatment, marketed as Hemlibra, includes the price of the drug and associated health system costs for administration in hospitals. The price per 30 mg vial of emicizumab is USD 551.2 (INR 40,754). For a 40 kg patient, 104 vials are typically needed, but due to a temporary discount from Roche Pharmaceuticals, only 56 vials are considered for cost calculation over a six-month maintenance phase. A scenario analysis was conducted to anticipate costs without the company’s discount. The health system costs were estimated based on the frequency of hospital visits, which is weekly for the loading dose and bi-monthly (2 visits/month) during the maintenance phase, totaling approximately 26 visits per year. [1]
Emicizumab is a cost-effective strategy
The research indicates that prophylaxis with emicizumab is a cost-effective strategy for severe haemophilia A patients with inhibitors in India, compared to on-demand treatment with bypassing agents (recombinant factor VIIa/aPCC). Emicizumab not only results in cost savings for the health system due to its negative Incremental Cost-Utility Ratio (ICUR) but also provides additional Quality-Adjusted Life Years (QALYs) for patients. The prophylactic use of emicizumab offers benefits such as fewer bleeding episodes, improved compliance with its once or twice monthly regimen, decreased healthcare costs due to its subcutaneous administration, and an enhanced quality of life for patients. [1]
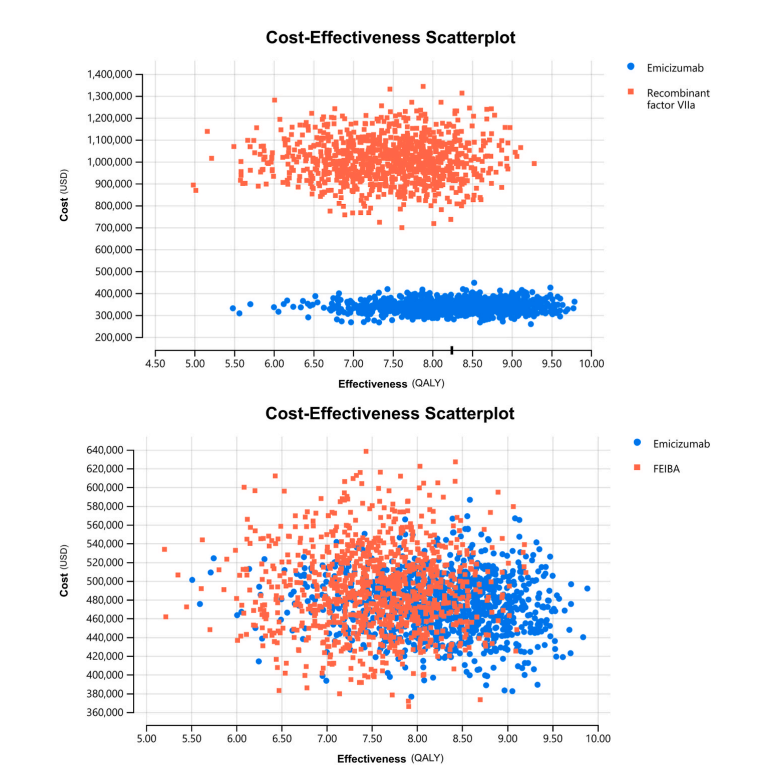
Source: Krishnamoorthy et al., 2024 [1]
The results, illustrated in Figure-2A, show that emicizumab not only reduces costs but also increases effectiveness, with the majority of the simulations positioning it in the Southeast quadrant of the cost-effectiveness plane, which signifies that the intervention is both less costly and more effective. Figure-2B supports these conclusions, showing the distribution of ICUR values across all four quadrants of the cost-effectiveness plane. Notably, about 63% of the simulations suggest that prophylactic emicizumab is likely to be a cost-saving option when compared to aPCC.[1]
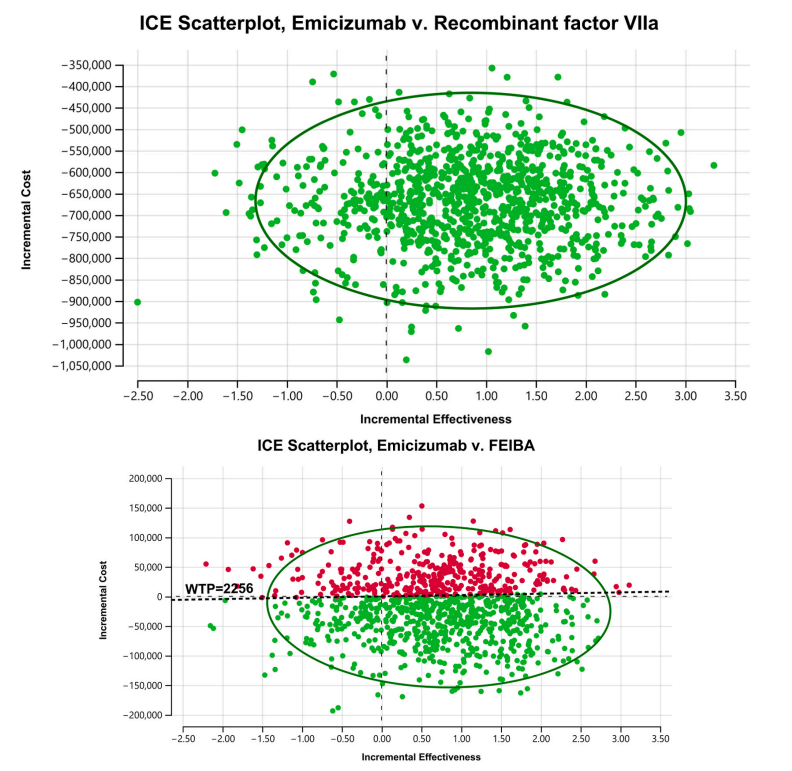
Source: Krishnamoorthy et al., 2024 [1]
Emicizumab prophylaxis compared to traditional on-demand therapy
Another study from India aimed to evaluate the cost-effectiveness of emicizumab prophylaxis in comparison to traditional on-demand therapy (ODT) and different dosing regimens of prophylaxis with FVIII, namely low dose prophylaxis (LDP), intermediate dose prophylaxis (IDP), and high dose prophylaxis (HDP), for the treatment of haemophilia A patients. The economic evaluation sought to determine the most efficient use of healthcare resources while maximising patient health outcomes. The economic evaluation took into account the varying practices in treatment across different economic settings, acknowledging that while HDP and IDP are prevalent in developed countries, ODT remains the standard in resource-limited settings, with LDP emerging as a viable alternative.
The above results showed emicizumab is cost-effective when compared to HDP and provides value for money when compared to ODT, IDP and LDP for severe haemophilia A in India. In doing the analysis, perspectives from the Indian payer, patient, and society were taken into consideration. Due to the absence of a predefined willingness-to-pay (WTP) threshold, the cost-effectiveness threshold was determined by the per-capita gross domestic product of the country in accordance with the recommendations provided by the Health Technology Assessment guidelines. [3]
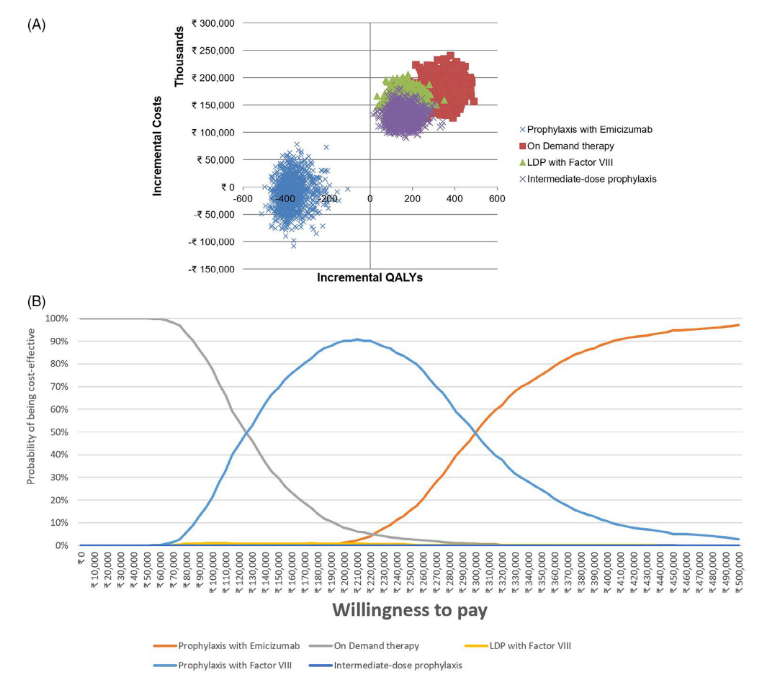
comparators
Source: Seth et al., 2024 [3]
Patient-Centered Outcomes and Quality of Life Considerations
The total estimated budget impact analysis for treating all existing haemophilia A patients in India over a 10-year period is approximately $59 million (INR 436 crores). This equates to an annual cost of $5.9 million (INR 43.6 crores). On a per patient basis, the 10-year treatment cost is $295,210 (INR 2.1 crores), translating to a negligible 0.0001% of India’s GDP annually. Considering India allocates up to 2% of its GDP for health, the cost for haemophilia A treatment represents a mere 0.0068% of the total healthcare budget. This data is crucial for regional and state level healthcare policymakers to assess the impact on the national health budget and for state health authorities to plan their healthcare expenditures both short and long term. [1]
The quality of life (QoL) in haemophilia A patients is adversely impacted by several interrelated factors:
Financial Condition: Due to the chronic nature of haemophilia and the ongoing need for expensive treatment, financial stability plays a crucial role in the wellbeing of haemophilia A patients. Those with limited financial resources are more prone to experience anxiety and distress, which can negatively impact their overall QoL.
Pain and Bleeding Frequency: The characteristic persistent bleeding episodes, especially in joints, lead to pain and can be frequent in some patients. This physical burden is directly associated with increased levels of anxiety and a diminished QoL.
Family and Societal Factors: The level of understanding and support from family and the broader community influences the psychological wellbeing of haemophilia patients. Families from less developed areas may have a deficient understanding of haemophilia, which can contribute to panic and an increased psychological burden. Moreover, haemophilia not only affects the patients but also has repercussions for their families, causing stress and anxiety at various levels. [2]
Conclusion
As we examine the multifaceted challenges and advancements in the management of haemophilia A, particularly in LMICs, several critical takeaways emerge:
Emicizumab has transformed the prophylactic treatment landscape for haemophilia A, offering a significant reduction in bleeding episodes and an improved quality of life for patients. However, its accessibility is uneven, with high-income countries enjoying greater access to this life-changing medication. Efforts must continue to bridge this gap and ensure that patients in LMICs can also benefit from such advancements in haemophilia care.
The economic evaluations conducted in India reveal that emicizumab is not only clinically effective but also cost-effective when compared to traditional on-demand therapies and various dosing regimens of Factor VIII prophylaxis. This finding underscores the importance of health economics in guiding healthcare policy and resource allocation, particularly in resource-constrained settings where maximising health outcomes within budgetary limits is crucial.
The burden of haemophilia A extends beyond the physical; it encompasses psychological, financial, and social dimensions that collectively impact patients’ QoL. A comprehensive approach to haemophilia care that includes psychosocial support, financial planning assistance, and community education is essential to address the full spectrum of needs faced by individuals with haemophilia A and their families.
References
- Krishnamoorthy Y, Govindan D, Kannan N, Majella MG, Hariharan VS, Valliappan V. Budget impact and cost-utility analysis of prophylactic emicizumab versus on-demand bypassing agents for adolescent severe haemophilia A patients with inhibitors in India. Heliyon. 2024 Mar 1;10(5):e27089. doi: 10.1016/j.heliyon.2024.e27089. PMID: 38468938; PMCID: PMC10926073.
- Wang X, Li Z, Li L. The hemophilia quality of life scale: a systematic review. Front Public Health. 2024 Feb 6;12:1294188. doi: 10.3389/fpubh.2024.1294188. PMID: 38379674; PMCID: PMC10876851.
- Seth T, John MJ, Chakrabarti P, Shanmukhaiah C, Verma SP, Radhakrishnan N, Dolai TK. Cost-effectiveness analysis of emicizumab prophylaxis in patients with haemophilia A in India. Haemophilia. 2024 Mar;30(2):426-436. doi: 10.1111/hae.14921. Epub 2023 Dec 26. PMID: 38147060.
- Abdelgawad HAH, Foster R, Otto M. Nothing short of a revolution: Novel extended half-life factor VIII replacement products and non-replacement agents reshape the treatment landscape in hemophilia A. Blood Rev. 2024 Mar;64:101164. doi: 10.1016/j.blre.2023.101164. Epub 2023 Dec 23. PMID: 38216442.
- Mehta P, Reddivari AKR. Hemophilia. [Updated 2023 Jun 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551607/
- Pezeshkpoor B, Oldenburg J, Pavlova A. Insights into the Molecular Genetic of Hemophilia A and Hemophilia B: The Relevance of Genetic Testing in Routine Clinical Practice. Hamostaseologie. 2022 Dec;42(6):390-399. doi: 10.1055/a-1945-9429. Epub 2022 Dec 22. PMID: 36549291; PMCID: PMC9779947.
- Mancuso ME, Croteau SE, Klamroth R. Benefits and risks of non-factor therapies: Redefining haemophilia treatment goals in the era of new technologies. Haemophilia. 2024 Mar 13. doi: 10.1111/hae.14976. Epub ahead of print. PMID: 38481077.
- Borhany M, Arshad A, Qureshi H, Nadeem R, Jamal A, Ahmed Khan R. Emicizumab Prophylaxis in Patients with Severe Hemophilia A: Insights from A Resource Limited Country. Clin Appl Thromb Hemost. 2024 Jan-Dec;30:10760296231224357. doi: 10.1177/10760296231224357. PMID: 38166474; PMCID: PMC10768607.
- Mannucci PM, Hermans C. Low-dose emicizumab for more equitable access to prophylaxis in resource limited countries. Haemophilia. 2024 Feb 28. doi: 10.1111/hae.14968. Epub ahead of print. PMID: 38415381.
Table of Contents
