Introduction:
The advent of biologic therapies has revolutionised the management of rheumatoid arthritis (RA), offering new hope for patients with severe symptoms. These advanced treatments target specific molecules in the immune system, effectively reducing inflammation and slowing disease progression. However, the increasing cost of biologics poses significant challenges for healthcare systems worldwide, raising critical questions about access, affordability, and long-term sustainability. In this Insight Article, we delve into the complex landscape of access and affordability of biologics in RA treatment, exploring their remarkable benefits, the budget impact they present, and potential strategies to strike a balance between optimal patient care and cost-effectiveness.
Unveiling the Promise of Biologics
Rheumatoid arthritis is a persistent, systemic, inflammatory autoimmune disorder that predominantly impacts synovial joints like those in the hands and feet. It results in swelling, stiffness, pain, and progressive irreversible damage to the joints. However, the disease can also manifest in other organs such as the lungs, heart, skin, and eyes. RA is linked with increased mortality and escalating disability, severely impacting quality of life and incurring significant costs from drug acquisition, hospitalisation, and decreased productivity.
There is currently no cure for RA but the goals in early stages is to suppress disease activity, induce remission, prevent functional loss, control joint damage and pain. RA treatment typically involves non-steroidal anti-inflammatory drugs (NSAIDs) or COX-2 inhibitors to alleviate pain, fever, joint swelling, and inflammation, and disease-modifying antirheumatic drugs (DMARDs) to mediate the immune response. Conventional DMARDs (such as methotrexate, leflunomide, and sulfasalazine) slow the disease progression and reduce joint damage.
Biological DMARDs
Biological DMARDs, which includes monoclonal antibodies and soluble receptors, can modify the disease process by blocking key protein messenger molecules (like cytokines) or cells (like B-lymphocytes). More recently added are targeted synthetic DMARDs (the Janus kinase (JAK) inhibitors) such as tofacitinib, baricitinib, filgotinib, and upadacitinib which play a crucial role in inflammation and autoimmune diseases including RA. [1]
Traditional DMARDs have been the cornerstone of treatment for newly diagnosed RA patients for a long time. The defining characteristic of a DMARD is its ability to slow down the clinical and radiographic progression of the disease. Therefore, the focus of RA treatment strategies has shifted towards early initiation of DMARDs to prevent structural joint damage and disability. Oral methotrexate (MTX) is most commonly used due to its long-term effectiveness, affordability, and acceptable safety profile, serving as the standard against which other DMARDs and newer RA therapies are measured.
American College of Rheumatology Guidelines
The American College of Rheumatology (ACR) has provided guidelines for safety monitoring for MTX and other DMARDs, particularly for patients with renal dysfunction who may be at a higher risk. The use of MTX in conjunction with other conventional DMARDs is on the rise, as research indicates that this approach may be more effective than single DMARD therapy in patients with early active RA. Unfortunately, DMARD therapy does not provide adequate response in some patients as remission is not achieved in 3 months or many do not maintain a response. This is where newer biologic treatments have emerged as important clinical alternatives in order to prevent irreversible joint damage.
Biologics have demonstrated significant efficacy in alleviating symptoms of RA, decelerating the advancement of the disease, and enhancing both physical functionality and life quality indicators. The clinical responses are typically swift, with the majority of patients noticing improvements within weeks of initiating therapy. [2] Biologics are a result of an improved understanding of RA pathogenesis. These engineered drugs target specific inflammatory cells, cellular interactions, and cytokines that are responsible for RA-related tissue damage. Their design aims to alleviate RA signs and symptoms and slow disease progression. The first biologic specifically targeted for RA, a tumor necrosis factor (TNF)-antagonist, etanercept (Enbrel®, Wyeth Pharmaceuticals), received approval from the US Food and Drug Administration (FDA) in 1998. Since then, several such agents have been made commercially available. [2]
The Rising Price Tag
The expanded variety of effective drugs and modes of action (MOAs), such as the integration of biologic drugs has significantly increased the chances of achieving treatment goals for RA patients, but the high costs associated with these drugs continue to restrict their universal application. Biologics are often expensive due to their complex manufacturing processes and research and development costs. The high cost of these medications poses challenges in terms of affordability and equitable access. Biologics are usually administered directly to the patient in a hospital or outpatient facility. This is because biologics are not in tablet/capsule form and therefore cannot be distributed by retail. The need for medical personnel to oversee patient treatment adds to the overall cost. In some countries, biologics are not subject to price regulation as they are dispensed in hospitals, leading to soaring prices. Furthermore, obtaining FDA approval for biologics is an arduous process. From 1982 to 2013, only 91 biologics received approval. This lengthy and complex approval process contributes to the high cost of these drugs. [3]
Improving affordability with biosimilars
The production of biosimilars, presents a significant challenge due to the intricate structure of biological drugs. Manufacturers often lack a comprehensive understanding of the biological processes involved in producing these drugs. This lack of insight makes it challenging for potential competitors to create less expensive biosimilars. This level of complexity is characteristic of all biologics production. The non-disclosure of these processes in patents further complicates the task of characterisation for potential competitors, making it nearly impossible. [3]
In the realm of drug pricing, biologics have been pinpointed as a significant contributor to the escalating costs. Despite constituting a mere 2% of US prescriptions in 2017, this small fraction was responsible for a staggering 37% of net drug spending. Moreover, biologics were responsible for 93% of net drug spending globally since 2014. The purchase rate of biologics is on an alarming upswing in the United States and other nations, particularly among those with the financial means to afford them.
In 2006, there was a 20% surge in biologics sales, equivalent to $40.3 million. This increase was in contrast to a modest 8% growth in total pharmaceutical product sales. The following year saw a 12.5% growth in biologics, approximately double the rate of the overall pharmaceutical market. A study conducted by Boston Consulting Group involving ten pharmaceutical companies revealed that the average production cost per pack for biologics was approximately $60. [3] Many healthcare systems have implemented cost-containment strategies, such as formulary restrictions, step therapy protocols, and biosimilar utilisation, to reduce expenditure while maintaining quality care
Balancing Cost-Effectiveness and Patient Care
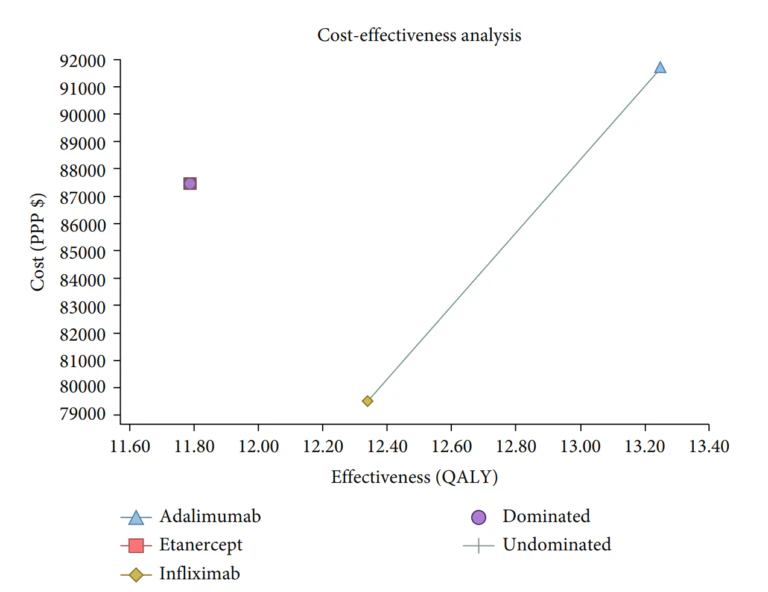
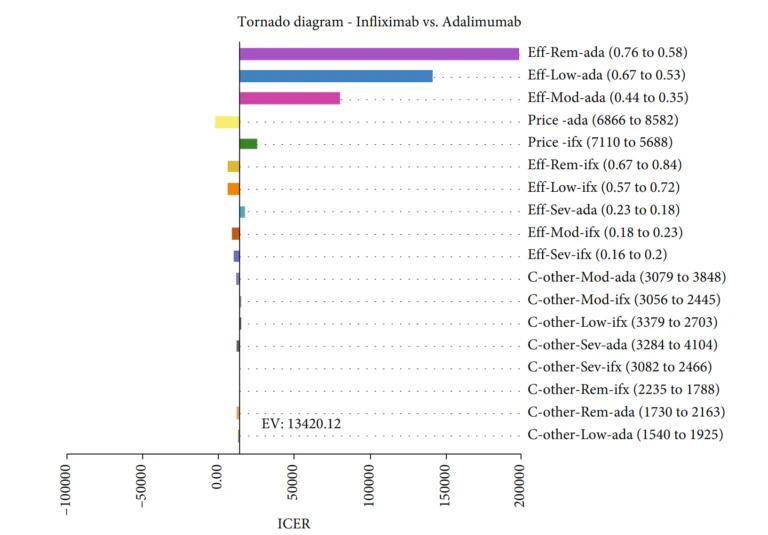
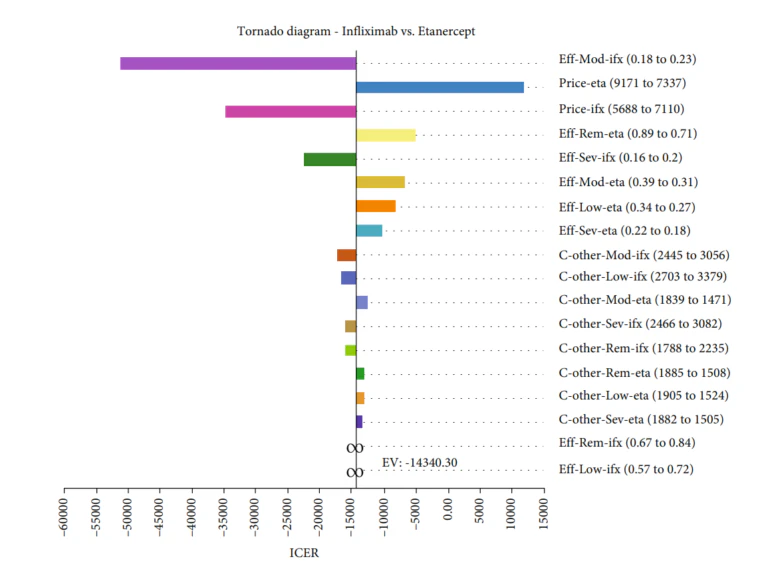
Gholami et al [4]conducted a study to assess the cost-effectiveness of various biologics, namely Infliximab, Adalimumab, and Etanercept, used in treating rheumatoid arthritis in Iran. A Markov model was employed to conduct this economic evaluation on 154 patients from Fars province. The findings showed that Infliximab had the lowest mean cost and a competitive QALY rate compared to the other two medications. The results showed that the mean costs and the QALY rates in the Infliximab, Adalimumab, and Etanercept arms were $ 79,518.33 and 12.34, $ 91,695.59 and 13.25, and $ 87,440.92 and 11.79, respectively. Furthermore, based on the probabilistic sensitivity analysis, Infliximab was deemed more cost-effective in 77% of simulations, making it the most cost-effective treatment option among the three.
Challenges in Accessibility and Affordability of Biologics
Numerous biologics used in RA treatment are nearing or have already reached patent expiry in many countries. As a result, the development of follow-on biologics, also known as ‘biosimilars’, is underway for RA treatment. A biosimilar can be described as a ‘biotherapeutic product that mirrors the quality, safety, and efficacy of an already licensed reference biotherapeutic product’. To obtain approval, a biosimilar must demonstrate a high degree of similarity to the original product in physicochemical and biological terms. Clinical studies are typically required to establish statistical equivalence in pharmacokinetics (PK) and efficacy, as well as to characterise the safety of the biosimilar. [5]
Biosimilars, which are highly similar to the original biologics, offer a potential cost-saving alternative. The introduction of biosimilar DMARDs has initiated a price competition, substantially reducing the net costs of biological DMARDs. However, this might not be the case in all countries and access to optimal care remains inadequate in low-income countries. [1]
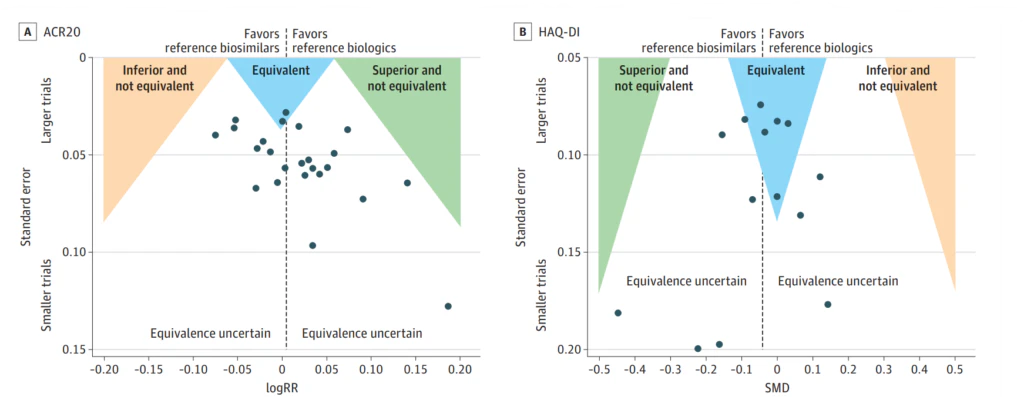
A comprehensive systematic review and meta-analysis published in 2023 has demonstrated convincing proof of parity between biosimilars of adalimumab, infliximab, and etanercept and their original counterparts in the use for RA. This was based on two critical parameter disease activity scores: the ACR20, a clinician-assessed outcome, and the HAQ-DI, a patient-reported outcome. These findings bolster a logical approach towards incorporating these biosimilars into RA treatment regimens. [6]
Future Directions and Policy Considerations
Both insurance companies and healthcare providers act as “purchasers” when it comes to biosimilars. Healthcare providers acquire biologics from manufacturers or wholesalers and then administer these drugs to patients. On the other hand, insurance companies exert influence over prescribing physicians by establishing their own reimbursement rates and utilising management tools like prior authorisation, which necessitates that prescribers furnish justification and documentation to validate the insurer’s payment for a particular drug.
In the recent years, biosimilars have emerged as a viable alternative to bio-originators, yet their unfamiliarity among patients and healthcare providers often leads to misconceptions about their quality. It is crucial to dispel these misconceptions by educating both patients and healthcare providers about the nature, approval process, and equivalent efficacy and safety of biosimilars compared to their bio-originators.
Approved biosimilars lower the costs of treatment
As the incidence of chronic diseases rises globally, regardless of income levels, there is a pressing need to transition towards more cost-effective pharmaceutical products to enhance access to necessary medications. Approved biosimilars present an opportunity to provide patients with an equivalent biologic at a lower cost than the bio-originator. The only distinguishing factor between a biosimilar and its bio-originator, assuming equal efficacy and safety, is the price.
The costs involved in the development of a biosimilar are significantly lower than those of a bio-originator. Therefore, once the patents for bio-originators expire, the introduction of more affordable biosimilars can help balance the costs of other necessary medications to meet unmet therapeutic needs. It is also important for payers to pass on the savings from the reduced cost of developing a biosimilar to the patients by enhancing access to treatment with lower copayments for medications or reduced insurance premiums.
A 2014 study by the RAND Corporation projected potential cost savings of $44.2 billion in the US market over the following decade from biosimilars, with TNF inhibitors contributing 21% ($9.3 billion) of these savings. In the European Union, the prices of biosimilars have typically been 20%-40% lower than those of the corresponding bio-originators. [7]
Key Take aways
- The advent of biologic therapies has revolutionised the management of rheumatoid arthritis (RA), offering new hope for patients with severe symptoms. However, the access and affordability of biologics poses significant challenges for healthcare systems worldwide, raising critical questions about access, affordability, and long-term sustainability.
- Biosimilars, which are highly similar to the original biologics, offer a potential cost-saving alternative. The introduction of biosimilar DMARDs has initiated a price competition, substantially reducing the net costs of biological DMARDs. However, access to optimal care remains inadequate in low-income countries.
- Despite the potential cost-saving benefits of biosimilars, there is a lack of understanding and awareness about these drugs among patients and healthcare providers, leading to hesitations in their use. Therefore, there is a pressing need to educate both patients and healthcare providers about the nature, approval process, and equivalent efficacy and safety of biosimilars compared to their bio-originators.
References:
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Müller-Ladner U, Mysler EF, da Silva JAP, Poór G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020 Jun;79(6):685-699. doi: 10.1136/annrheumdis-2019-216655. Epub 2020 Jan 22. PMID: 31969328.
- Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011 Jun;33(6):679-707. doi: 10.1016/j.clinthera.2011.05.044. PMID: 21704234; PMCID: PMC3707489. https://pubmed.ncbi.nlm.nih.gov/21704234/
- Favour Danladi Makurvet, Biologics vs. small molecules: Drug costs and patient access, Medicine in Drug Discovery, Volume 9, 2021, 100075, ISSN 2590-0986, https://doi.org/10.1016/j.medidd.2020.100075. https://www.sciencedirect.com/science/article/pii/S2590098620300622
- Gholami A, Azizpoor J, Aflaki E, Rezaee M, Keshavarz K. Cost-Effectiveness Analysis of Biopharmaceuticals for Treating Rheumatoid Arthritis: Infliximab, Adalimumab, and Etanercept. Biomed Res Int. 2021 Nov 28;2021:4450162. doi: 10.1155/2021/4450162. PMID: 34877355; PMCID: PMC8645365. https://pubmed.ncbi.nlm.nih.gov/34877355/
- Yoo DH, Prodanovic N, Jaworski J, Miranda P, Ramiterre E, Lanzon A, Baranauskaite A, Wiland P, Abud-Mendoza C, Oparanov B, Smiyan S, Kim H, Lee SJ, Kim S, Park W. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017 Feb;76(2):355-363. doi: 10.1136/annrheumdis-2015-208786. Epub 2016 Apr 29. PMID: 27130908; PMCID: PMC5284338.
- Ascef BDO, Almeida MO, Medeiros-Ribeiro ACD, Oliveira de Andrade DC, Oliveira Junior HAD, de Soárez PC. Therapeutic Equivalence of Biosimilar and Reference Biologic Drugs in Rheumatoid Arthritis: A Systematic Review and Meta-analysis. JAMA Netw Open. 2023;6(5):e2315872. doi:10.1001/jamanetworkopen.2023.15872 https://pubmed.ncbi.nlm.nih.gov/37234004/
- Kay J, Schoels MM, Dörner T, Emery P, Kvien TK, Smolen JS, Breedveld FC; Task Force on the Use of Biosimilars to Treat Rheumatological Diseases. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis. 2018 Feb;77(2):165-174. doi: 10.1136/annrheumdis-2017-211937. Epub 2017 Sep 2. PMID: 28866648.
Table of Contents

